Technology
Scientists Unveil Groundbreaking Technology to Track Cell Messages
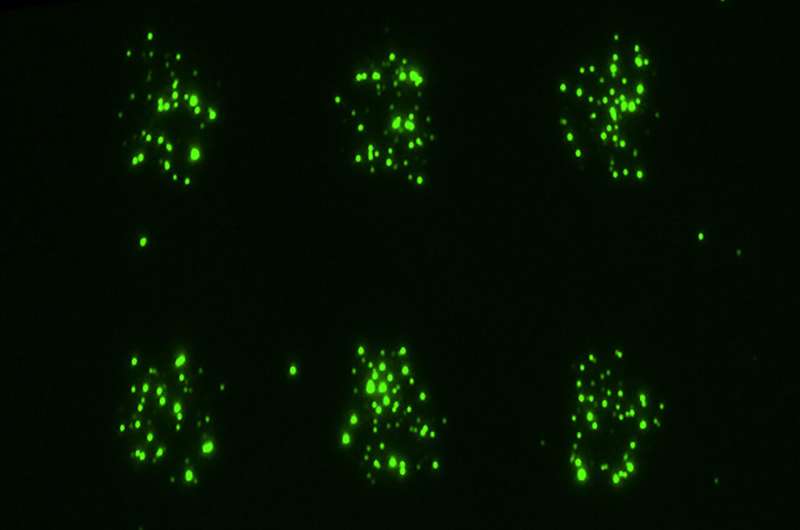
Researchers from Northwestern University and The Ohio State University have introduced a pioneering technology that allows scientists to observe how cells communicate through tiny biological packages known as extracellular vesicles and particles (EVPs). This new tool, called LEVA (light-induced extracellular vesicle and particle adsorption), enables precise arrangements of EVPs, which cells release to convey messages within the body.
The study, published in Nature Methods on November 18, 2025, showcases impressive time-lapse videos in which biological nanoparticles navigate across a surface, forming distinct patterns while guided by chemical signals. This innovative method provides insights into how EVPs influence critical biological processes, including wound healing, immune responses, and cancer progression.
Understanding Extracellular Vesicles
EVPs play a vital role as communicators between cells, carrying essential molecular cargo such as proteins and RNA. These nanoparticles are naturally released into biofluids and tissues, where they contribute to various physiological functions. Traditionally, research focused on EVPs suspended in liquid; however, the LEVA technology allows for a new approach by fixing these vesicles in place, creating a roadmap for observing cellular behavior.
Colin Hisey, an assistant professor of biomedical engineering at Northwestern, co-led the research. He noted, “Our research provides scientists with a powerful new tool to understand how cells communicate through the ‘breadcrumb trails’ they leave behind during movement in both healthy and disease contexts.” This tool could pave the way for novel treatment strategies targeting diseases and enhancing healing processes.
Mechanism and Applications of LEVA
LEVA operates by using ultraviolet light to create a stencil-like pattern on a surface. This process alters the chemical properties of the exposed areas, making them adhesive to EVPs while leaving other areas neutral. As a result, scientists can generate controlled patterns of EVPs—such as dots, lines, or intricate shapes—mimicking their natural arrangement in human tissues.
Hisey highlighted the importance of EVPs in cancer migration, wound healing, and immune responses, stating, “Previously, scientists lacked the tools to study them quantitatively and systematically. LEVA uses controlled ultraviolet light to attract these vesicles with subcellular precision based on their innate properties.” This advancement comes at a crucial juncture as research in this area gains momentum.
In experiments, researchers created patterns of bacterial EVPs and introduced human neutrophils—white blood cells that act as first responders in the immune system. The neutrophils quickly identified and clustered around these EVPs, mimicking their behavior during actual infections. The resulting time-lapse videos vividly portray how immune cells respond to the chemical signals emitted by EVPs, reinforcing their role as beacons for immune response.
“Neutrophils have evolved to recognize the antigens present on bacterial cells and, hence, also bacterial EVPs because they are so similar,” Hisey explained. This platform offers a unique opportunity to delve deeper into the dynamics of immune signaling and inflammation.
Looking ahead, Hisey and his team aim to expand LEVA’s applications beyond flat surfaces. They plan to adapt the technology for three-dimensional materials to better replicate the complex conditions found within the human body. The team intends to investigate how different types of EVPs influence cell behavior across various disease contexts, with initial focuses on cancer metastasis, wound healing, and immune responses to pathogenic EVPs.
Ultimately, the goal is to develop therapeutic strategies that either enhance or inhibit the communications mediated by these vesicles, along with exploring the interactions of nanoparticles with surfaces from a materials engineering perspective. “Our long-term objectives include systematically mapping how different types of surface-bound vesicles affect cell behavior in various conditions and configurations,” Hisey added.
This research not only advances scientific understanding but also holds potential implications for future therapies that could improve health outcomes across a range of medical challenges.
-

 Top Stories1 week ago
Top Stories1 week agoPiper Rockelle Shatters Record with $2.3M First Day on OnlyFans
-

 Top Stories4 days ago
Top Stories4 days agoMeta’s 2026 AI Policy Sparks Outrage Over Privacy Concerns
-

 Top Stories7 days ago
Top Stories7 days agoUrgent Update: Denver Fire Forces Mass Evacuations, 100+ Firefighters Battling Blaze
-

 Top Stories1 week ago
Top Stories1 week agoOnlyFans Creator Lily Phillips Reconnects with Faith in Rebaptism
-

 Entertainment4 days ago
Entertainment4 days agoTom Brady Signals Disinterest in Alix Earle Over Privacy Concerns
-

 Top Stories5 days ago
Top Stories5 days agoOregon Pilot and Three Niece Die in Arizona Helicopter Crash
-

 Sports3 days ago
Sports3 days agoLeon Goretzka Considers Barcelona Move as Transfer Window Approaches
-

 Top Stories2 days ago
Top Stories2 days agoCBS Officially Renames Yellowstone Spin-off to Marshals
-

 Health2 months ago
Health2 months agoTerry Bradshaw Updates Fans on Health After Absence from FOX NFL Sunday
-

 World1 week ago
World1 week agoTragedy in Crans-Montana: Fire Claims Lives of Holiday Revelers
-

 Sports2 days ago
Sports2 days agoSouth Carolina Faces Arkansas in Key Women’s Basketball Clash
-

 World1 week ago
World1 week agoFire Claims Lives at New Year’s Party in Swiss Ski Resort

