MIT Researchers Uncover Genome’s 3D Structure During Cell Division
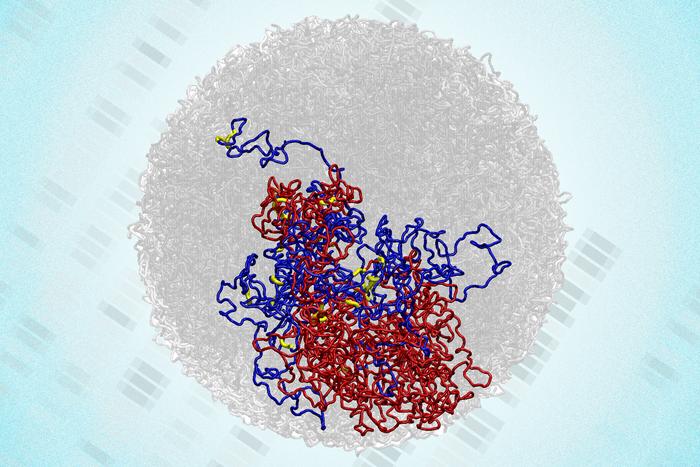
Recent research led by scientists at the Massachusetts Institute of Technology (MIT) has challenged a long-standing belief regarding the genome’s structure during cell division. Traditionally, it was thought that during mitosis, the genome loses its distinctive three-dimensional (3D) arrangement, only to regain it after the process concludes. However, findings published in the journal Natural Structural & Molecular Biology reveal that certain 3D structures persist even as cells divide.
The research team utilized a cutting-edge technique known as Region-Capture Micro-C (RC-MC), which offers a higher-resolution mapping of the genome than previous methods. This innovative approach allowed the researchers to identify that small 3D loops connecting regulatory elements and genes remain intact during mitosis. These loops appear to strengthen as chromosomes compact in preparation for division, enabling cells to “remember” interactions from one cell cycle to the next.
Anders Sejr Hansen, PhD, an associate professor of biological engineering at MIT, emphasized the significance of these findings, stating, “In the past, mitosis was thought of as a blank slate, with no transcription and no structure related to gene activity. Now we know that there’s always structure. It never goes away.” This insight bridges the gap between genome structure and its functionality in regulating gene expression, a long-standing challenge in genetics.
The study, which features lead author Viraat Goel, PhD, along with co-senior authors Hansen and Edward Banigan, PhD, demonstrates that the 3D structure and function of the genome are interconnected throughout the cell cycle. As chromosomes undergo reorganization during mitosis, larger structures like A/B compartments and topologically associating domains (TADs) disappear. Yet, the newly identified microcompartments persist and may even strengthen during this phase.
Over the past two decades, researchers have recognized that DNA within the cell nucleus organizes into intricate 3D loops. While many of these loops facilitate interactions between genes and regulatory regions, others contribute to chromosome compaction during cell division. Previous research primarily utilized the Hi-C technique, which, despite being groundbreaking, lacked the resolution necessary to reveal the details of these specific interactions.
In 2023, Hansen and his team developed the RC-MC method, achieving a resolution up to 1,000 times greater than Hi-C. This advancement enables focused analysis on targeted genome regions, significantly enhancing the mapping of 3D structures. The researchers now have a clearer understanding of how microcompartments form during cell division.
The study shows that as chromosomes compact, one might expect microcompartments to vanish. Surprisingly, the researchers observed that these structures remain visible and even gain prominence during mitosis. “Unexpectedly, we observe microcompartments in mitosis, in contrast to all prior Hi-C studies reporting that chromosomes lose all 3D genome structural patterns during cell division,” the authors noted.
The implications of these findings are significant. Leonid Mirny, PhD, a professor at MIT and a co-author of the study, highlighted that these microcompartments may explain the increased gene transcription that occurs near the end of mitosis. Historically, it was believed that transcription halts completely during this phase, but recent studies have indicated a brief spike in transcription activity.
The research team found that during mitosis, these microcompartments are often located near genes that experience this transcriptional spike. This suggests that the compaction of the genome during mitosis brings enhancers and promoters closer together, facilitating the formation of microcompartments. Hansen remarked, “It almost seems like this transcriptional spiking in mitosis is an undesirable accident that arises from generating a uniquely favorable environment for microcompartments to form.”
As the cell transitions into the G1 phase after mitosis, many of these microcompartments weaken or disappear. This raises questions about how cells determine which structures to maintain and which to discard. The researchers aim to explore how variations in cell size and shape might influence genome structure and gene regulation.
This groundbreaking study provides new insights into the complexities of genome organization during cell division, emphasizing that the genome’s 3D structure is not merely a transient feature but an integral part of cellular function throughout the cell cycle. The findings open new avenues for understanding gene regulation, potentially reshaping our knowledge in the field of genetics.