Scientists Harness Light to Transform Greenhouse Gases into Chemicals
Researchers from China have developed an innovative method to convert greenhouse gases, specifically carbon dioxide (CO2) and methane (CH4), into valuable chemicals using only light, eliminating the need for costly catalysts. This groundbreaking study, published in the journal Nature Photonics on December 14, 2025, reveals that high-energy photons can directly break the chemical bonds in these gases, presenting a potential solution to climate change.
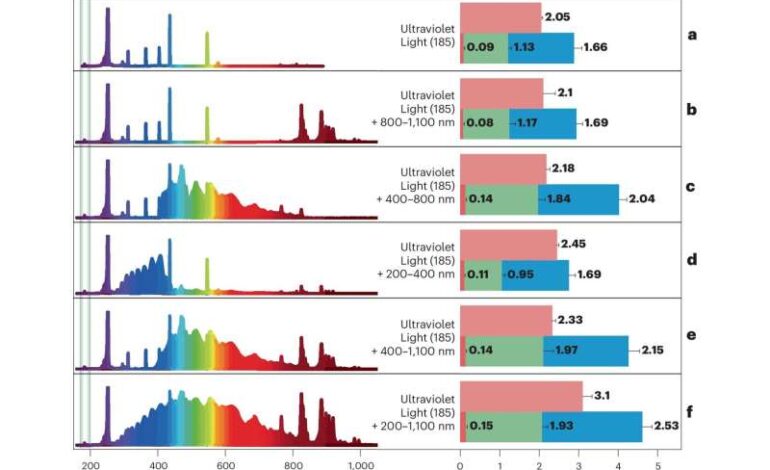
The team utilized a specialized 28-W ultraviolet light source emitting photons with a wavelength of 185 nm. This light effectively disrupted the strong molecular bonds found in methane and carbon dioxide, enabling the conversion of these gases into various chemicals, including water gas (CO/H2) and ethane (C2H6). Remarkably, this reaction occurred under ambient conditions, as well as in oxygen-free environments that mimic outer space.
The urgency of this research stems from the significant role of carbon dioxide and methane in global warming. Together, these gases account for nearly 84% of the rise in global temperatures, contributing to severe climate impacts and ocean acidification, which poses threats to marine ecosystems worldwide. As traditional methods for reducing greenhouse gas emissions focus primarily on limiting their release, scientists are increasingly exploring ways to capture and repurpose these gases.
Converting CO2 and CH4 into other molecules has long been a challenge due to their stable chemical structures. Conventional techniques often necessitate high temperatures exceeding 700 °C and high pressures, accompanied by the use of expensive metal catalysts, rendering the processes energy-intensive and costly. The breakthrough achieved by this team of researchers circumvents these barriers by using light to initiate the transformation.
The process began with the construction of a quartz reactor chamber filled with a mixture of 99.9% pure carbon dioxide and methane. The chamber was subjected to different types of light under low pressure and maintained at a controlled temperature of 25°C. The targeted light wavelength activated the otherwise inert gas molecules, enabling the conversion process.
Results from gas analysis indicated that the light-driven reactions produced carbon monoxide, hydrogen, and ethane at production rates of 3.1 mmol m-3 h-1, 1.93 mmol m-3 h-1, and 2.53 mmol m-3 h-1, respectively. Further experiments showed that introducing water into the mixture and eliminating atmospheric oxygen improved yields. In a simulated space-like environment, where the reaction chamber was flushed with argon gas, the researchers achieved a total gas conversion of 1.51% within 24 hours.
While the current yield remains modest, the researchers assert that their findings highlight a promising new approach to utilize greenhouse gases as resources rather than waste. This method not only offers a pathway to mitigate climate change but also fosters the development of a circular economy where emissions are transformed into valuable products without the need for extreme energy demands or costly catalysts.
In conclusion, this study marks a significant advancement in the quest for sustainable solutions to climate change. By leveraging the power of light, scientists have opened new avenues for converting greenhouse gases into useful chemicals, offering hope for a cleaner and more sustainable future.