Scientists Discover New Ice Form at Room Temperature
Researchers have identified a new type of ice, known as ice XXI, which forms under unique conditions at room temperature. This discovery, made by scientists from the Korea Research Institute of Standards and Science (KRISS), adds to the existing knowledge of water’s complex behavior and its ability to exist in various forms.
Traditionally, ice is viewed simply as frozen water, but it can take on more than 20 distinct structures, each defined by its atomic arrangement. Some forms, like the familiar ice found in freezers, are common, while others only emerge under extreme conditions, such as high pressure in the Earth’s mantle or on distant celestial bodies. Understanding these forms not only satisfies scientific curiosity but also enhances our knowledge of environments where life might exist beyond Earth.
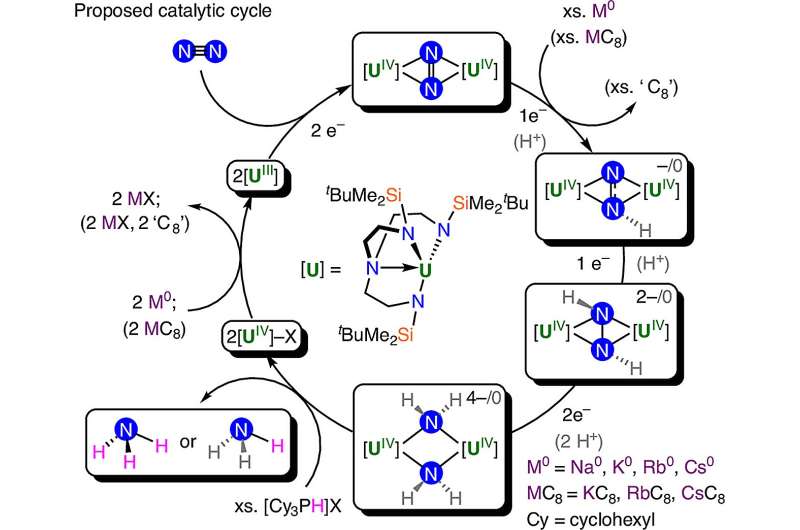
In their study, researchers focused on the behavior of super-compressed water, utilizing advanced technology that includes diamond anvils and X-ray lasers. This innovative setup allowed them to observe the freezing process of water in real time, revealing that instead of crystallizing in a straightforward manner, water underwent multiple freeze-melt cycles. These cycles occur within the pressure range where ice VI typically forms, leading to the emergence of ice XXI.
Unique Characteristics of Ice XXI
Ice XXI is distinguished by its unique atomic structure, which sets it apart from over 20 previously known ice types. Notably, it is metastable, meaning it can persist in an unstable form for a time under specific conditions. This characteristic provides valuable insights into how ice can form under varying pressures, potentially informing studies of icy planets and deep Earth environments.
Geun Woo Lee, a scientist with KRISS, highlighted the significance of their findings: “Rapid compression of water allows it to remain liquid up to higher pressures, where it should have already crystallized to ice VI.” This research utilized diamond anvil cells to create high-pressure conditions, where ultra-pure water was placed in a thin metal chamber. By employing fast cameras and laser-based sensors, the team meticulously monitored the freezing and melting processes.
The researchers employed X-ray beams from a synchrotron to capture the critical moments of water transitioning into ice. They adjusted the pressure rhythm during the experiment to gain a better understanding of the freezing process. Two types of detectors were used to record the transformation at varying speeds, enabling them to analyze the intricate dynamics of water molecules as they froze.
Implications for Future Research
The study also involved molecular dynamics simulations using two models: SPCfw45 and TIP4P/Ice46. Although one model is rigid and the other allows for flexibility, both demonstrated similar trends in how water reacts under pressure. Their findings revealed that when water is subjected to super-compression at room temperature, it does not freeze in a single step but rather follows a series of freeze-melt loops before stabilizing into ice VI.
Ice XXI specifically forms at a pressure of approximately 1.6 gigapascals and exhibits a body-centered tetragonal crystal structure. It is noteworthy that this new ice form has more energy than MS-ice VII at room temperature, indicating it is less stable. This discovery opens the door to understanding complex ice behavior; for instance, only ice XXI can transition into MS-ice VII, while regular water cannot.
The research team also discovered that water can crystallize along multiple pathways, rather than a single route, even at room temperature. “With the unique X-ray pulses of the European XFEL, we have uncovered multiple crystallization pathways in H2O,” Lee explained. Fellow researcher Rachel Husband added, “These findings suggest that a greater number of high-temperature metastable ice phases and their associated transition pathways may exist, potentially offering new insights into the composition of icy moons.”
The full study detailing these groundbreaking findings has been published in the journal Nature Materials. This research not only contributes to our understanding of water’s behavior under extreme conditions but may also have implications for exploring extraterrestrial environments where similar forms of ice could exist.