Researchers Uncover Mechanism for Embryonic Development in Fruit Flies
A study conducted by biologists at Dartmouth College has revealed significant insights into how embryos transition during early development. Published in EMBO Reports, the research focuses on fruit flies and identifies the signals prompting embryos to shift from rapid cell division to the organization of specialized cells. This discovery could enhance understanding of similar processes in human development.
After fertilization, embryos undergo a series of rapid cell divisions before slowing down to form distinct cell types vital for various functions within the body. The precise signals that trigger this transition have remained largely elusive. Senior author Amanda Amodeo, an assistant professor of biological sciences, emphasized the importance of the process: “The way that the DNA is packaged changes pretty dramatically in the very early embryo, as it goes from maternal control to transcribing its own genes.”
The research team demonstrated that this crucial change is linked to the embryo’s nuclear-to-cytoplasmic ratio, a factor that may provide insights into certain cancers and aging in humans, which are often characterized by disruptions in this ratio.
Fruit Flies Serve as Key Research Model
Fruit flies, or Drosophila, have long been favored in embryonic development studies due to their transparent embryos and extensive genetic tools available for researchers. The embryos, which are about the size of a needle tip, hatch within 24 hours, allowing for rapid observation and experimentation.
The early stages of embryonic development are largely governed by maternal proteins inherited from the mother, which direct the embryo’s processes before it begins to produce its own proteins. After approximately 13 rounds of synchronized cell division, the single nucleus expands to over 6,000 nuclei, prompting a change in control from maternal to embryonic oversight.
Amodeo and her team investigated the role of histone proteins in this transition. Histones are critical for DNA organization, protecting it from damage, and facilitating gene activation. Previous studies indicated that specific histones are associated with gene transcription in adult cells, but their impact on embryonic development remained unclear.
The research showed that the histone variant H3.3 replaces histone H3 when the embryo detects a high concentration of nuclei. This transition is crucial for moving the embryo into a new phase of development.
Significance of Histone Variants
First author Anusha Bhatt, a PhD student in the Guarini School of Graduate and Advanced Studies, explained the functional differences between H3 and H3.3: “Even though they only differ by four amino acids, H3 and H3.3 have very different functions.” H3 is primarily involved in genome duplication, whereas H3.3 is utilized in regions where gene transcription is necessary.
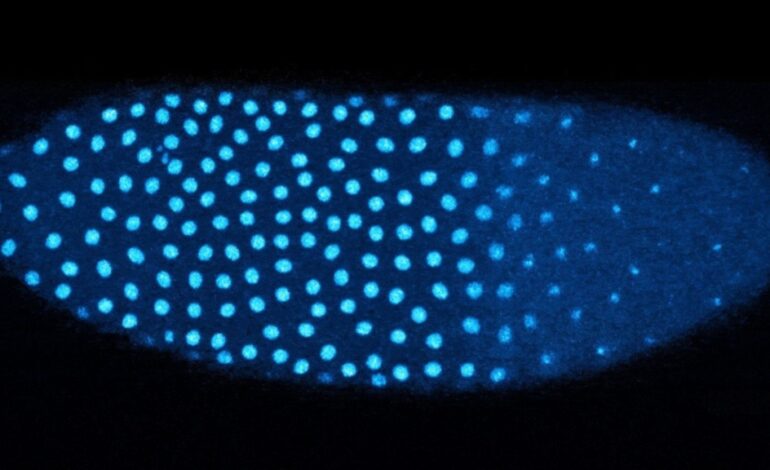
The Dartmouth researchers used fluorescent tagging to track the presence of H3 and H3.3 in fruit fly embryos as they approached the critical 13th round of cell division. Their findings revealed that while the amount of H3 decreased with each division, the levels of H3.3 increased significantly.
Crowded regions within the embryos showed a higher incorporation of H3.3, indicating that the distribution of nuclei plays a vital role in activating genes necessary for further development. In contrast, uncrowded regions did not exhibit the same transition, highlighting the importance of nuclear density in this process.
Looking ahead, the research team aims to explore the role of H3.3 in genome activation further. Bhatt expressed interest in determining where H3.3 is incorporated within the genome during early cell divisions and which genes it influences.
The study also acknowledges the contributions of two undergraduate students, Madeleine Brown and Aurora Wackford, who have participated in this research. “I’m very proud of both of them and this work,” Amodeo stated, recognizing their efforts as they contribute to the expanding field of developmental biology.
This research not only enhances the understanding of embryonic development in fruit flies but may also pave the way for breakthroughs in human health, particularly in understanding the mechanisms behind developmental disorders and diseases linked to gene activation.