Researchers Uncover Key Protein Folding Mechanism in Cells
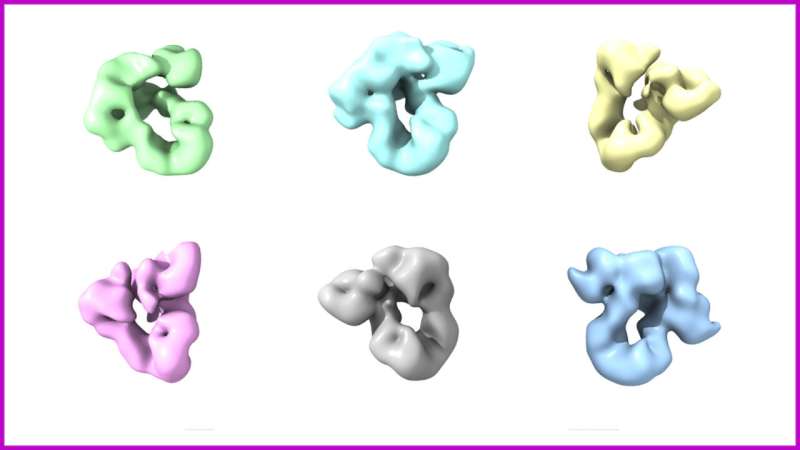
Research conducted at the Center for Medical Biotechnology (ZMB) at the University of Duisburg-Essen has unveiled a vital cellular mechanism that governs protein folding, a process essential for proper cellular function and overall health. Published on July 21, 2025, in the journal Nature Structural & Molecular Biology, this study focuses on the BiP–GRP94 chaperone complex, which plays a critical role in ensuring proteins achieve their correct three-dimensional structures within the endoplasmic reticulum, the cell’s main production and quality control center.
At the forefront of this significant research, Prof. Dr. Doris Hellerschmied, the senior author of the publication, emphasized the groundbreaking nature of their findings. “Our study is the first to reveal at the structural level how the chaperones BiP and GRP94 work together,” she stated. The research team demonstrated that the chaperone complex undergoes sequential and coordinated changes while performing its function, highlighting the importance of its conformational plasticity in recognizing and processing diverse protein folding states.
Advancements in Understanding Protein Folding
Utilizing advanced techniques such as high-resolution electron microscopy and biochemical analyses, the researchers visualized previously unknown conformations of the BiP–GRP94 complex. These discoveries shed light on the functional significance of these structural changes. According to Dr. Simon Pöpsel, co-lead author of the study, “Our results provide valuable insights into the cell’s molecular machinery.”
The implications of this research extend beyond fundamental biology. Disruptions in protein folding have been linked to various serious health conditions, including certain neurodegenerative disorders. The insights gained from this study could pave the way for developing innovative therapeutic approaches aimed at addressing these diseases.
This research marks a significant step forward in our understanding of cellular processes and may ultimately contribute to advancements in medical treatments for conditions where protein folding is compromised.
The study is authored by Joel Cyrille Brenner and colleagues, highlighting the collaborative effort among researchers to explore this critical aspect of cellular function. The findings underscore the complexity of molecular biology and the ongoing efforts to unravel the intricate processes that sustain life.
As our understanding of these mechanisms deepens, the potential for breakthroughs in medical science becomes increasingly promising, with the hope of improving health outcomes for individuals affected by protein misfolding disorders.






