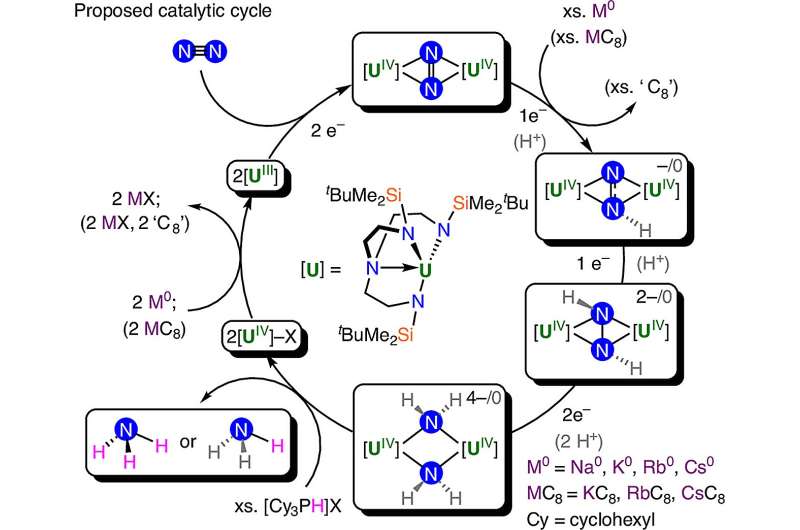
Pfizer’s mRNA Flu Vaccine Shows Promise Amid Approval Concerns
Pfizer’s experimental mRNA-based flu vaccine has demonstrated significant effectiveness in early trials, but its future approval in the United States faces uncertainty due to ongoing skepticism surrounding mRNA technology. According to data published on October 18, 2023, in the New England Journal of Medicine, the vaccine, known as modRNA, showed a marked improvement over traditional flu vaccines during a Phase III trial involving over 18,000 participants across the United States, South Africa, and the Philippines during the 2022-2023 flu season.
The study, funded by Pfizer, revealed that those who received the modRNA vaccine reported significantly fewer confirmed cases of influenza compared to those who received the standard Fluzone vaccine, produced by Sanofi Pasteur. Specifically, there were 57 confirmed cases in the modRNA group versus 87 cases in the control group, leading to an overall effectiveness increase of 34.5% in preventing flu-like illness.
Trial Details and Findings
Participants in the trial were randomly assigned to receive either the modRNA vaccine or Fluzone, with both vaccines targeting the four most common strains of influenza anticipated for that season. The results indicated that the modRNA vaccine elicited a higher antibody response against influenza A strains and a comparable response to influenza B strains. The researchers noted, “This randomized trial showed that the modRNA vaccine provided both similar and improved prevention of a first episode of laboratory-confirmed influenza in adults between the ages of 18 and 64 years.”
While the findings are promising, there are caveats. Individuals receiving the modRNA vaccine reported more local reactions, such as injection site pain, with 70.1% experiencing such effects compared to 43.1% for Fluzone. Additionally, systemic adverse events, including fever, were more prevalent in the modRNA group (5.6% vs. 1.7%). Despite these findings, the adverse effects were generally mild or moderate, leading researchers to conclude that the overall safety profiles of both vaccines were similar.
Regulatory Challenges Ahead
Despite the positive trial results, the path to regulatory approval for the modRNA vaccine is fraught with challenges. Concerns have arisen from figures such as Robert F. Kennedy Jr., the current U.S. Secretary of Health and Human Services, who has historically expressed skepticism towards vaccines, particularly those based on mRNA technology. His comments and those of his allies have contributed to a climate of distrust surrounding vaccines, particularly since the onset of the COVID-19 pandemic.
Recently, the Centers for Disease Control and Prevention (CDC) updated its website to include controversial claims regarding vaccines, reinforcing fears that have been debunked by scientific research. Criticism from anti-vaccination groups has included unfounded assertions that mRNA vaccines are linked to serious health issues, including cancer. Such sentiments have real-world implications, as evidenced by Moderna’s decision to delay the approval application for its combination flu and COVID-19 vaccine earlier this year, following requests for additional data from the Food and Drug Administration (FDA).
In August, Kennedy also withdrew $500 million in federal funding earmarked for mRNA vaccine research and development, further complicating the landscape for this technology. These actions reflect a growing resistance to mRNA vaccines, which many believe could enhance public health efforts against seasonal flu.
Despite these hurdles, the advantages of mRNA technology, such as quicker adaptation to circulating flu strains, present a compelling case for its use. Pfizer is expected to seek approval for the modRNA vaccine in the near future, alongside Moderna, which has indicated plans to submit for approval of its standalone flu shot next year. Given the study’s promising results, both vaccines could potentially have a strong chance of receiving regulatory approval, although the current climate remains unpredictable.
As vaccine technology continues to evolve, the implications of these trials could shape the future of flu vaccination efforts worldwide, emphasizing the need for clear communication and understanding of mRNA vaccines among the public.