New Uranium Catalyst Transforms Air Nitrogen into Ammonia Efficiently
Scientists at the Ecole Polytechnique Federale de Lausanne have developed a groundbreaking uranium-based catalyst that efficiently converts air nitrogen into ammonia. This innovation, published in the journal Nature Chemistry on July 16, 2025, represents a significant advancement in sustainable ammonia production methods, critical for agricultural fertilizers.
Currently, ammonia production primarily relies on the Haber-Bosch process, which transforms nitrogen gas (N2) from the atmosphere into ammonia. This traditional method is energy-intensive and generates considerable greenhouse gas emissions. Researchers have long sought alternatives that could streamline this process while minimizing environmental impacts.
According to Professor Marinella Mazzanti at EPFL, existing molecular catalysts typically bind nitrogen molecules—composed of two nitrogen atoms—to a single metal center in a linear arrangement. This limits the efficiency of the reaction. In contrast, nature employs a multimetallic approach, allowing nitrogen molecules to bind flexibly to multiple metals, facilitating easier bond breaking.
Innovative Approach Inspired by Nature
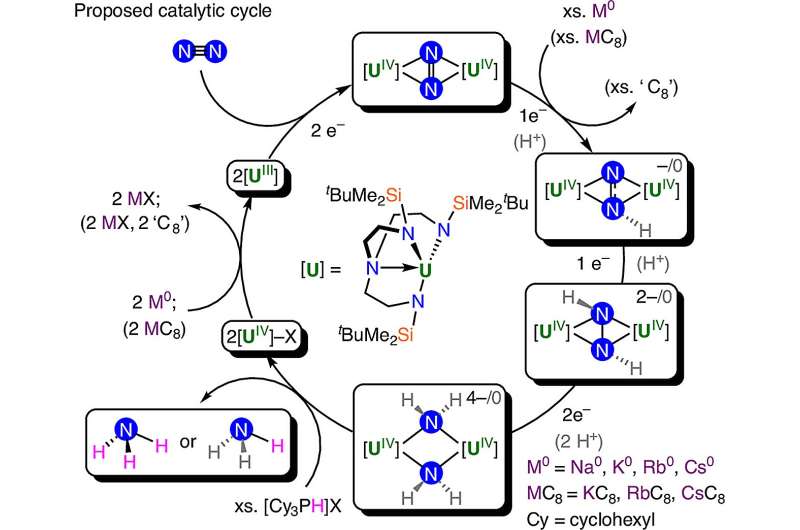
The research team, led by Mazzanti, has successfully created the first molecular uranium catalyst capable of binding nitrogen gas in a “side-on” configuration. This method mimics biological processes found in nature, particularly those involving enzymes known as nitrogenases, which efficiently convert nitrogen into ammonia.
The scientists synthesized a unique molecular complex using uranium combined with a triamidoamine ligand. This combination allows the catalyst to hold nitrogen gas sideways, enabling a more effective conversion process. Through a stepwise reduction of nitrogen gas, the team was able to break the strong bond between the two nitrogen atoms, producing intermediate forms such as N2 2-, N2 3-, and N2 4-, ultimately resulting in the formation of separate nitride ions (N3-).
Promising Results for Sustainable Ammonia Production
The experiments demonstrated that the uranium complex could be cycled repeatedly, achieving a conversion rate of up to 8.8 equivalents of ammonia per uranium catalyst. This finding provides compelling evidence that side-on nitrogen binding, akin to the mechanisms employed by nature, can be a viable pathway for ammonia production.
This discovery sheds light on previously unknown steps in nitrogen conversion chemistry and reinforces the potential of uranium as a catalyst in industrial applications. Historically, uranium was one of the first metals used for ammonia production, and this research suggests that its capabilities may still be underutilized.
The implications of this work extend beyond mere scientific advancement. Enhanced methods for ammonia production could play a crucial role in addressing global food security by providing a more sustainable and efficient means of fertilizer production. As the world grapples with increasing agricultural demands, innovations like this uranium catalyst offer a hopeful glimpse into the future of sustainable farming practices.
More information on this research can be found in the article: Batov, M.S., et al. “Catalytic and stoichiometric stepwise conversion of side-on bound di-nitrogen to ammonia mediated by a uranium complex,” published in Nature Chemistry (2025). DOI: 10.1038/s41557-025-01867-z.






